My challenging toxic subject is the molecule. Not any particular nanoscale unit of potentially hazardous substance, nor any particular substance in the aggregate. Rather, I am interested in the material and historical grounds for the peculiar idea that molecules and only molecules may be the proximate causes of (chemical) toxicity. This principle that a toxin is a chemical is a molecule defines the stage on which most modern dramas of toxic chemical perceptibility and epistemology play out. It sustains, among other things, the perverse pattern of "regrettable substitution" (whence this essay's title), also known as "chemical whack-a-mole"—the replacement of a hazardous substance by a substitute that turns out to be just as toxic. The origins of this history lie in visualization: the late 19th century convention (much the same today) of representing chemical substances using doodles of letters, representing atoms, and lines, representing bonds. The principle of toxin-as-chemical-as-molecule came about through the transformation of such doodles into fixed chemical names and notation. Deployed within the authoritative information technologies of chemistry, from many-volume print reference works to present-day digital databases, this molecular vocabulary ordered the chemical world for the convenience of chemists and, especially, the synthetic chemicals industry. I would like to reverse this process. By foregrounding the contingency of the molecule as toxic agent, I wish to open space for remapping environmental toxicity along dimensions new and forgotten, such as that of 19th century Alsatian chemist Charles Gerhardt, for whom chemicals were beings “defined by their metamorphoses, that is, by their past or by their future."

My challenging toxic subject is the molecule. Not any particular nanoscale unit of potentially hazardous substance, nor any particular substance in the aggregate. Rather, I am interested in the material and historical grounds for the peculiar idea that molecules and only molecules may be the proximate causes of (chemical) toxicity. This principle that a toxin is a chemical is a molecule defines the stage on which most modern dramas of toxic chemical perceptibility and epistemology play out. It sustains, among other things, the perverse pattern of "regrettable substitution" (whence this essay's title), also known as "chemical whack-a-mole"—the replacement of a hazardous substance by a substitute that turns out to be just as toxic. The origins of this history lie in visualization: the late 19th century convention (much the same today) of representing chemical substances using doodles of letters, representing atoms, and lines, representing bonds. The principle of toxin-as-chemical-as-molecule came about through the transformation of such doodles into fixed chemical names and notation. Deployed within the authoritative information technologies of chemistry, from many-volume print reference works to present-day digital databases, this molecular vocabulary ordered the chemical world for the convenience of chemists and, especially, the synthetic chemicals industry. I would like to reverse this process. By foregrounding the contingency of the molecule as toxic agent, I wish to open space for remapping environmental toxicity along dimensions new and forgotten, such as that of 19th century Alsatian chemist Charles Gerhardt, for whom chemicals were beings “defined by their metamorphoses, that is, by their past or by their future."
Evan Hepler-Smith
Boston College
Substantive Caption:
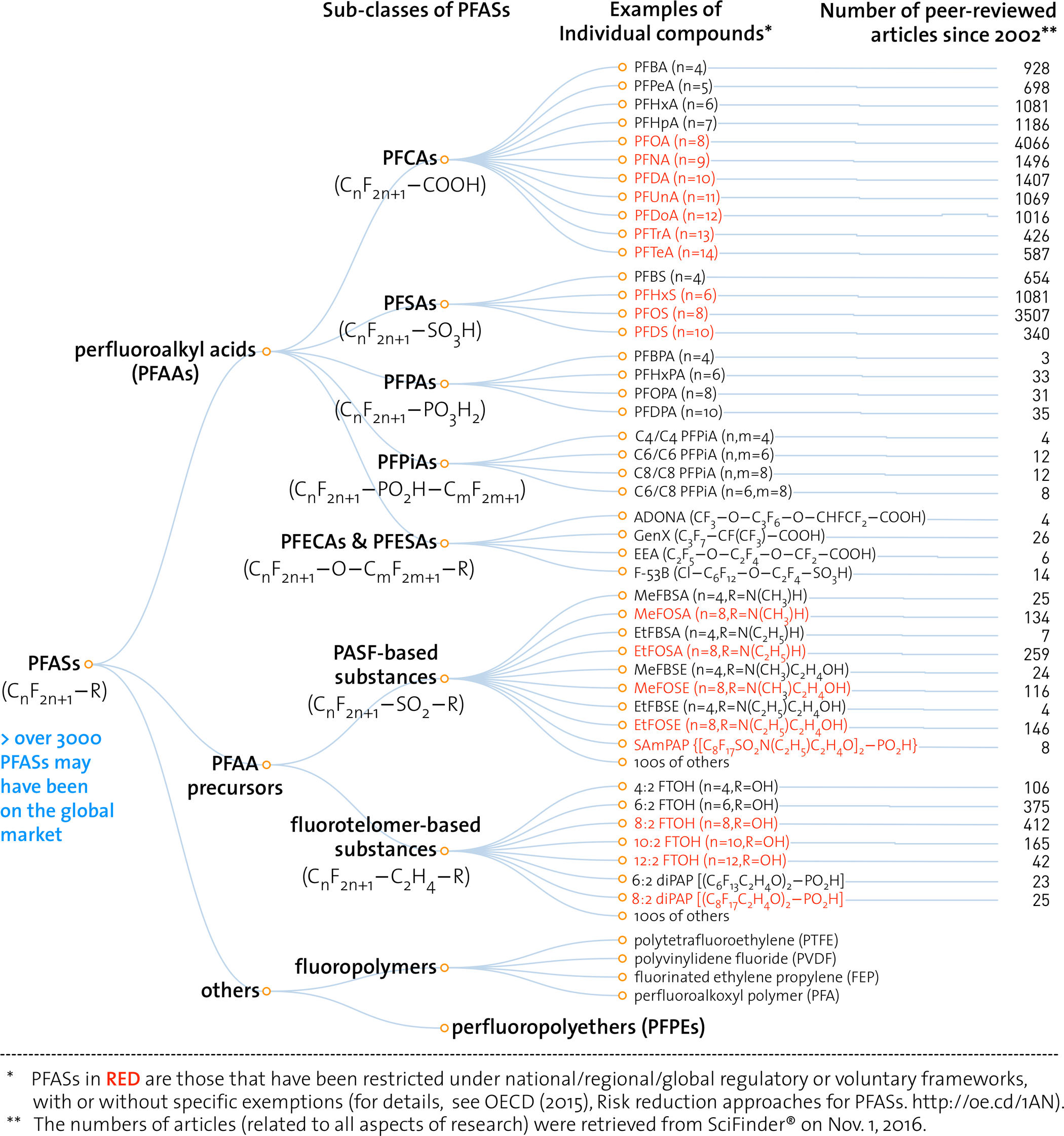
“PFASs as a whole, much more than solely PFOS, PFOA, and their precursors, are an intractable, potentially never-ending chemicals management issue” (Wang 2017, 2511).
This image depicts the “family tree” of the chemicals PFOS (perfluoroctasulfonic acid) and PFOA (perfluorooctanoic acid). PFOA and PFOS are persistent environmental chemicals associated with a range of toxic effects including increased incidence of liver disease, thyroid disease, high cholesterol, decreased vaccine efficacy, decreased fertility, pre-eclampsia, and several types of cancer (ATSDR 2018). PFOS—formerly the key ingredient in the stain-resistant coating Scotchgard—is among the two-dozen persistent organic pollutants restricted or banned under the UN’s Stockholm Convention. PFOA—a key constituent in Teflon production until the mid-2000s—is under consideration for addition to the Stockholm Convention in April 2019.
As these two toxic chemicals have been phased out, they have been replaced, Hydra-like, by diverse related chemicals from the broader class of PFCs or PFASs (perfluoro chemicals / per- and polyfluoroalkyl substances). Environmental toxicologists are concerned that the same structural similarities that make other PFASs useful substitutes for the industrial uses of PFOA and PFOS may also make the entire class just as hazardous as these two substances that have become major public health concerns. This figure, from a 2017 review article addressing PFAS toxicology, is intended to illustrate the molecule-by-molecule breadth of this toxic hazard and the comparatively narrow scope of research and regulation. The molecule-by-molecule detail of the figure is overwhelming, inscrutable, frustrating, intractable. That is the point. The authors wish to argue that, as long as environmental science, law, and politics hew to molecule-by-molecule conceptions of what's toxic, the task of controlling environmental toxicity will remain overwhelming, inscrutable, frustrating, and intractable, too.
The authors of this article explain, “The most common current industrial practice of phasing out one PFAS is to replace it with another (or multiple other) structurally similar PFAS. Such a strategy is easier and less costly than identifying a nonfluorinated substance to be used in the same or similar process (i.e., chemical replacement) or inventing a new process that does not require PFASs (i.e., functionality replacement).…. [B]ut such a replacement strategy will not solve issues in relation to PFASs as a whole group—it will only increase the numbers of PFASs on the market and the difficulties in tracking them” (Wang 2017).
This image portrays environmental toxicology and toxic substances regulation as trapped in a molecular double bind. On the one hand, the quantity of studies dedicated to PFOA and PFOS attest to how much work it takes to begin to understand the long-term, low-dose toxicity of a specific chemical compound—let alone to take action to address it. On the other hand, the molecule-by-molecule list of PFASs at right illustrates how focusing on specific compounds risks missing the chemical forest for the molecular trees. And yet this forest—the white noise of ubiquitous multiple chemical exposure (Murphy 2006)—is precisely the white noise that makes it take so much work to pick out a toxic signal associated with an individual substance in the first place.
In sum, according to this figure, there are too many molecules to know, and it’s very hard to know anything at all about any of them, in large part because there are so many of them to know. This is a fruitful starting point for asking historical and ethnographic questions about the ontology of the agents of environmental toxicity. Where did we get the idea that the environment (or the hazardous anthropogenic bits of it, anyway) is made up of molecules? What is a molecule? Are there alternative ontologies on offer, within or outside of the chemical and toxicological sciences?
One place to start: The impression that there is just too much to know is a general feature of the “informating of environmentalism” (Fortun 2004). Historically, such concerns have tended to emerge in the wake of novel technologies that afford new practices and imaginaries and scales of information management (Blair 2010).
Design Statement:
In this essay, I take data visualization and image-based arguments as subject matter for historical and ethnographic analysis. My particular interest is in the relationship between, on the one hand, genres and conventions for making and interpreting images, and on the other hand, databases and reference works containing data that gets visualized in such images. By attending to the connection between information infrastructure (Bowker and Star 1999) and image-making, we can investigate the interdependence between the ways data gets stored and the “ways in which [a community of practice] attunes to, interacts with, and shapes its objects in its various and varied practices.” (Mol 2002, vii; see also Hacking 2002). To oversimplify: visualization method + data structure = ontology.
Methodologically, this essay aims to model the sort of approach that ethnographers and historical ethnographers might apply reflexively to assess their own methods of data visualization and image-making—especially methods drawing on data and techniques developed within other fields. My work shows how, as chemical databases and visualization methods are taken up outside their fields of origin, cross-disciplinary user communities can fall into a “certainty trough” (Mackenzie 1990), taking inscriptions created as index terms (where can you find data about a certain substance?) and granting them ontological status (what substance is this data about?). This is not necessarily a bad way of enacting the world, but we should be aware that we’re doing it!
Cover image
I want to avoid the temptation of getting lost in a hall of mirrors of representation and re-mediation, when it comes to this screenshot of a database-accessed copy of a digital scan of a newsmagazine article with a figure reproducing a soft-focus photograph of a chemical typewriter typing a diagrammatic representation of a chemical substance that never was. Instead, I want to emphasize the proliferation of articles containing figures like this in mid-20th century chemical journals, extolling the capacity of this or that chemical typewriter to type legible and interpretable structural formulas rather than hand-drawing them (or employing a professional graphical artist to do so). Across pen-and-paper, chalk-and-slate, mechanical, and digital media, the genre of the structural formula representing the molecular structure of a chemical substance has remained remarkably consistent since the late 1860s. Digital ethnographers might ask whether phenomena of interest are best explained in terms of digital media, in terms of genre, and/or along other dimensions of continuity and change.
This image
The taxonomic tree is a common visual genre for expressing a collection of relationships. Taxonomic trees reflect, imply, or constitute a hierarchical classification, pinning down entities to specific positions within it. Yet these trees also afford a certain flexibility, permitting the viewed to lump or split, emphasize sameness or difference, depending on which specific taxa or taxonomic level one chooses to privilege. Indeed, natural historians have at times interpreted trees as illustrations of the unreality of species as natural kinds. By this view, what’s arboreal is arbitrary.
Rhetorically, such images are frequently employed to overwhelm, confronting the viewer with a surfeit of complexity, employing an all-encompassing order to emphasize the incomprehensible scope of specifics. The tree in this figure, for instance, presents an impression of order-cum-overload supporting the article authors’ argument that the variety of fluorine-rich synthetic chemical substances subsumed in this hierarchy is in part responsible for their “Never-Ending Story” (per the article’s title) as persistent organic pollutants.
References:
ATSDR (Agency for Toxic Substances and Disease Registry, United States Department of Health and Human Services). 2018. Toxicological Profile for Perfluoroalkyls (Draft for Public Comment). https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf.
Blair, Ann. 2010. Too Much to Know: Managing Scholarly Information before the Modern Age. New Haven: Yale University Press.
Bowker, Geoffrey C., and Susan Leigh Star. 1999. Sorting Things Out: Classification and Its Consequences. Cambridge, MA: MIT Press.
Fortun, Kim. 2004. “From Bhopal to the Informating of Environmentalism: Risk Communication in Historical Perspective.” Osiris 19: 283–96. doi: 10.1086/649407.
Hacking, Ian. 2002. Historical Ontology. Cambridge, MA: Harvard University Press.
Mackenzie, Donald A. 1990. Inventing Accuracy: A Historical Sociology of Nuclear Missile Guidance. Cambridge, Mass: MIT Press.
Mol, Annemarie. 2002. The Body Multiple: Ontology in Medical Practice. Durham: Duke University Press.
Murphy, Michelle. 2006. Sick Building Syndrome and the Problem of Uncertainty: Environmental Politics, Technoscience, and Women Workers. Durham: Duke University Press.
Wang, Zhanyun et al. 2017. “A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?.” Environmental Science & Technology 51 (5): 2508–18. doi: 10.1021/acs.est.6b04806.
(Revision of May 9, 2019)
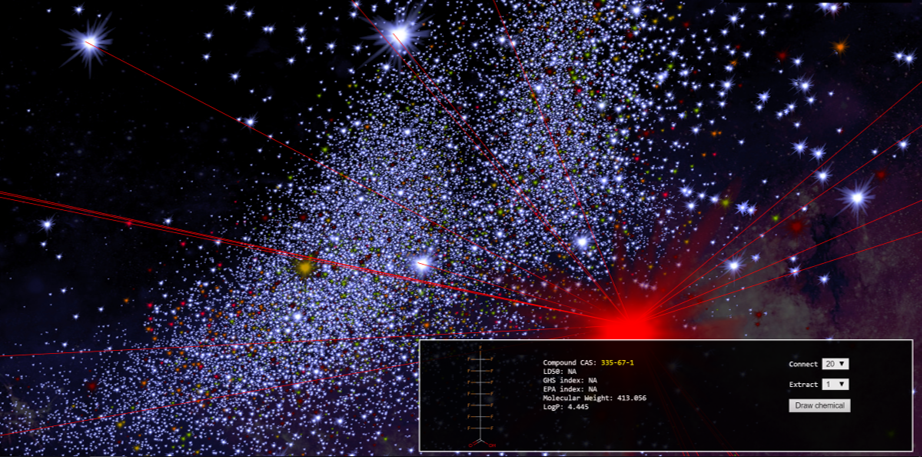
Substantive Caption:
Screen capture from the web app ChemMaps, depicting a view of “chemical space” from the perspective of PFOA. Each “star” in this map represents a chemical compound of a distinct molecular identity. The relative positions of these stars indicate structural similarity, according to “a complex compendium of 1D, 2D and 3D pre-computed molecular descriptors to generate the chemical space in three dimensions” (Borrel, Kleinstreuer, and Fourches 2018). Based on the assumption that chemical properties are correlated with molecular structure—a bedrock of modern organic chemistry—the proximity of substances in this map is supposed to be an index of chemical and toxicological similarity. Like most work in cheminformatics, ChemMaps was originally designed to expedite drug discovery. However, “ChemMaps.com aims to become the go-to website for anyone wanting to search, mine or visualize chemical space” (Borrel, Kleinstreuer, and Fourches 2018). This includes those concerned about environmental toxicity of chemicals like the PFASs.
Here, hovering just over the shoulder of PFOA, the viewer floats in a purely molecular galaxy. This chemical holism purports to represent the constituents of the material world of Teflon and Scotchgard and cancer. However, this form of visualization unintentionally dramatizes how far removed this holism of molecules is from the world of materials. It is one realization of a trend that the philosopher François Dagognet associated with the representation of chemical substances in abstract molecular terms: “whereas the poets have always suffered from the gap between things and signs, chemistry tranquilly effects the miracle of their coincidence. The neologisms, however, lose their attachment with sensible reality, qualities, and appearances. In order to be able to go to the depths of substances, on the ocean of their relationships, it’s necessary to break the moorings. The learned words, kinds of algebraic polynomials, cease then to touch us. Uprooted, they address themselves only to the intelligence of structures.” (Dagognet 2002 [1969], 158).
Framing the problem of environmental toxicity as a problem of information overload tends to suggest computational solutions. Molecules are extremely well adapted to computer modeling and large-scale comparisons. But the intensification of computational methods is suspiciously analogous to the intensified production of new, putatively safer chemicals as a solution to environmental toxicity. It may be that the computational tractability of molecules is an index of their intractability as toxic subjects. Perhaps here, as in other domains, “the simplification of ontology has led to the enormous complication of epistemology,” (Viveiros de Castro 2004).
Design Statement:
This image is a data visualization expressing calculated estimates of toxicity-relevant properties of chemical substances. Like most (all?) visualizations, it is a visual metaphor: here, chemical substances are stars, and spatial proximity is toxicological similarity. As Lily Kay (2000) points out, scientific metaphors have ontological force – the ways they render the world may beget ways of interacting with and remaking it. Molecular structures are abstractions, not material substances; one might define chemical identity in many other ways. The visual metaphor of the star map enforces a conception of chemical individuality that strongly privileges the molecular structure – each molecule, forever alone and itself, in the chemical heavens.
Two methodological points: first, ethnographers may wish to reflect on the implications of the visual metaphors they employ in data visualization and other image-making practices. Second, if we wish to create ethnographic images that contest and situate putative views from nowhere, we should be careful to avoid (or at minimum ironize) data visualizations practices that reproduce such a view.
References:
Borrel, Alexandre, Nicole C. Kleinstreuer, and Denis Fourches. 2018. “Exploring Drug Space with ChemMaps.Com.” Bioinformatics 34 (21): 3773–75. doi: 10.1093/bioinformatics/bty412.
Dagognet, François. 2002 [1969]. Tableaux et langages de la chimie : essai sur la représentation. Seyssel: Champ Vallon.
Kay, Lily E. 2000. Who Wrote the Book of Life?: A History of the Genetic Code. Stanford: Stanford University Press.
Viveiros de Castro, Eduardo Batalha. 2004. “Exchanging Perspectives: The Transformation of Objects into Subjects in Amerindian Ontologies.” Common Knowledge 10 (3): 463–484. doi: 10.1215/0961754X-10-3-463.
(Revision of May 9, 2019)
Substantive Caption:
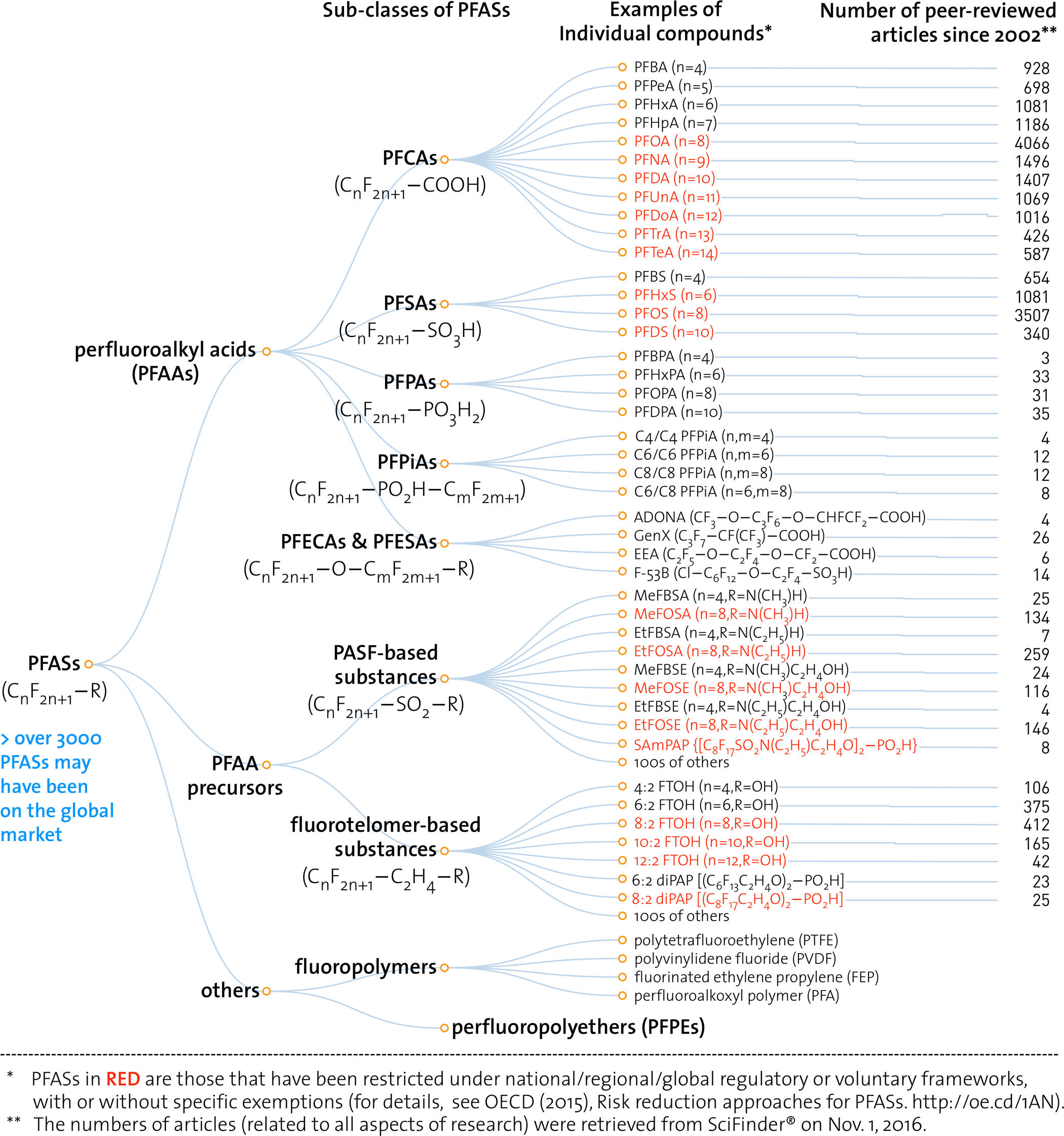
“PFASs as a whole, much more than solely PFOS, PFOA, and their precursors, are an intractable, potentially never-ending chemicals management issue” (Wang 2017, 2511).
This image depicts the “family tree” of the chemicals PFOS (perfluoroctasulfonic acid) and PFOA (perfluorooctanoic acid). PFOA and PFOS are persistent environmental chemicals associated with a range of toxic effects including increased incidence of liver disease, thyroid disease, high cholesterol, decreased vaccine efficacy, decreased fertility, pre-eclampsia, and several types of cancer (ATSDR 2018). PFOS—formerly the key ingredient in the stain-resistant coating Scotchgard—is among the two-dozen persistent organic pollutants restricted or banned under the UN’s Stockholm Convention. PFOA—a key constituent in Teflon production until the mid-2000s—is under consideration for addition to the Stockholm Convention in April 2019.
As these two toxic chemicals have been phased out, they have been replaced, Hydra-like, by diverse related chemicals from the broader class of PFCs or PFASs (perfluoro chemicals / per- and polyfluoroalkyl substances). Environmental toxicologists are concerned that the same structural similarities that make other PFASs useful substitutes for the industrial uses of PFOA and PFOS may also make the entire class just as hazardous as these two substances that have become major public health concerns. This figure, from a 2017 review article addressing PFAS toxicology, is intended to illustrate the molecule-by-molecule breadth of this toxic hazard and the comparatively narrow scope of research and regulation.
The authors of this article explain, “The most common current industrial practice of phasing out one PFAS is to replace it with another (or multiple other) structurally similar PFAS. Such a strategy is easier and less costly than identifying a nonfluorinated substance to be used in the same or similar process (i.e., chemical replacement) or inventing a new process that does not require PFASs (i.e., functionality replacement).…. [B]ut such a replacement strategy will not solve issues in relation to PFASs as a whole group—it will only increase the numbers of PFASs on the market and the difficulties in tracking them” (Wang 2017).
Design Statement:
This image portrays environmental toxicology and toxic substances regulation as trapped in a molecular double bind. On the one hand, the quantity of studies dedicated to PFOA and PFOS attest to how much work it takes to begin to understand the long-term, low-dose toxicity of a specific chemical compound—let alone to take action to address it. On the other hand, the molecule-by-molecule list of PFASs at right illustrates how focusing on specific compounds risks missing the chemical forest for the molecular trees. And yet this forest—the white noise of ubiquitous multiple chemical exposure (Murphy 2006)—is precisely the white noise that makes it take so much work to pick out a toxic signal associated with an individual substance in the first place.
In sum, according to this figure, there are too many molecules to know, and it’s very hard to know anything at all about any of them, in large part because there are so many of them to know. This is a fruitful starting point for asking historical and ethnographic questions about the ontology of the agents of environmental toxicity. Where did we get the idea that the environment (or the hazardous anthropogenic bits of it, anyway) is made up of molecules? What is a molecule? Are there alternative ontologies on offer, within or outside of the chemical and toxicological sciences?
One place to start: The impression that there is just too much to know is a general feature of the “informating of environmentalism” (Fortun 2004). Historically, such concerns have tended to emerge in the wake of novel technologies that afford new practices and imaginaries and scales of information management (Blair 2010).
References:
ATSDR (Agency for Toxic Substances and Disease Registry, United States Department of Health and Human Services). 2018. Toxicological Profile for Perfluoroalkyls (Draft for Public Comment). https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf.
Blair, Ann. 2010. Too Much to Know: Managing Scholarly Information before the Modern Age. New Haven: Yale University Press.
Fortun, Kim. 2004. “From Bhopal to the Informating of Environmentalism: Risk Communication in Historical Perspective.” Osiris 19: 283–96. doi: 10.1086/649407.
Murphy, Michelle. 2006. Sick Building Syndrome and the Problem of Uncertainty: Environmental Politics, Technoscience, and Women Workers. Durham: Duke University Press.
Wang, Zhanyun et al. 2017. “A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?.” Environmental Science & Technology 51 (5): 2508–18. doi: 10.1021/acs.est.6b04806.
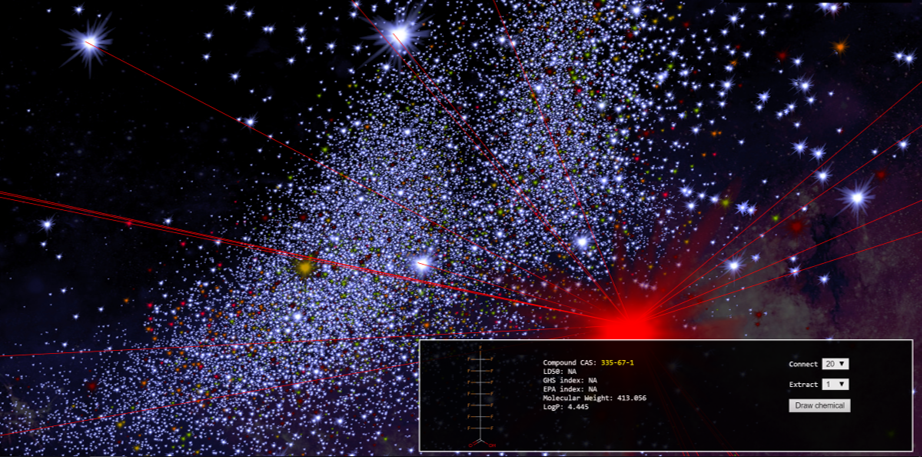
Substantive Caption:
Screen capture from the web app ChemMaps, depicting a view of “chemical space” from the perspective of PFOA. Each “star” in this map represents a chemical compound of a distinct molecular identity. The relative positions of these stars indicate structural similarity, according to “a complex compendium of 1D, 2D and 3D pre-computed molecular descriptors to generate the chemical space in three dimensions” (Borrel, Kleinstreuer, and Fourches 2018). Based on the assumption that chemical properties are correlated with molecular structure—a bedrock of modern organic chemistry—the proximity of substances in this map is supposed to be an index of chemical and toxicological similarity. Like most work in cheminformatics, ChemMaps was originally designed to expedite drug discovery. However, “ChemMaps.com aims to become the go-to website for anyone wanting to search, mine or visualize chemical space” (Borrel, Kleinstreuer, and Fourches 2018). This includes those concerned about environmental toxicity of chemicals like the PFASs.
Here, hovering just over the shoulder of PFOA, the viewer floats in a purely molecular galaxy. This chemical holism purports to represent the constituents of the material world of Teflon and Scotchgard and cancer, but this form of visualization dramatizes how far away we are. It is one realization of a trend that the philosopher François Dagognet associated with the representation of chemical substances in abstract molecular terms: “whereas the poets have always suffered from the gap between things and signs, chemistry tranquilly effects the miracle of their coincidence. The neologisms, however, lose their attachment with sensible reality, qualities, and appearances. In order to be able to go to the depths of substances, on the ocean of their relationships, it’s necessary to break the moorings. The learned words, kinds of algebraic polynomials, cease then to touch us. Uprooted, they address themselves only to the intelligence of structures.” (Dagognet 2002 [1969], 158).
Design Statement:
References:
Borrel, Alexandre, Nicole C. Kleinstreuer, and Denis Fourches. 2018. “Exploring Drug Space with ChemMaps.Com.” Bioinformatics 34 (21): 3773–75. doi: 10.1093/bioinformatics/bty412.
Dagognet, François. 2002 [1969]. Tableaux et langages de la chimie : essai sur la représentation. Seyssel: Champ Vallon.
Viveiros de Castro, Eduardo Batalha. 2004. “Exchanging Perspectives: The Transformation of Objects into Subjects in Amerindian Ontologies.” Common Knowledge 10 (3): 463–484. doi: 10.1215/0961754X-10-3-463.

Substantive Caption:
The main image depicts the editorial offices of Chemical Abstracts, circa 1938. For most of the 20th century and through the present-day, the abstract journals, collective indexes, and databases of Chemical Abstracts (now Chemical Abstracts Service, or CAS) have been the authoritative source for bibliographic and regulatory information about chemical substances and the chemical sciences, including allied fields such as toxicology. In this image, “index workers” are engaged in compiling an annual or decennial index of the publication. The vast majority of these cards refer to individual chemical substances rather than subject headings or author names. Much index labor involved writing, rewriting, cross-referencing, and error-checking the systematic chemical names that were the key to the whole enterprise, since they allowed for the arrangement of hundreds of thousands (today hundreds of millions) of substances in chemically-meaningful alphabetical order. This work was particularly challenging because the research chemists who wrote the journal articles abstracted and indexed in Chemical Abstracts often did not themselves use systematic names. This was both because the names were cumbersome and because the uniform chemical ontology that they expressed was sometimes a poor fit for how chemists defined the substances that their research addressed.
“Among personal characteristics for this type of work accuracy perhaps should rank highest, and with that conscientiousness, patience, a meticulous attentiveness to detail (without a loss of perspective of relative values, since a great deal of work has to be turned out, at times under considerable pressure), power of concentration, good judgment, and interest in words as words, a love of puzzles and of guessing and digging out elusive ideas, meanings, words, and formulas. An interest in things rather than people is likely to lead to greater satisfaction in this type of work, since the opportunities for personal professional contacts outside the office are relatively few. The analytical rather than the creative type is probably best suited to this kind of work. Good eyes (at least strong ones) are absolutely essential, while the ability to sit hour after hour without much relief is a requirement not to be laughed off. Work of this nature is not for the overly energetic or restless person” (Scott 1938, 275; italics in original).
The inset image depicts a form of the sort that was used when CAS re-created this whole operation on digital computers during the 1960s.
Design Statement:
Molecular identity is not given by nature. Rather, it was made in meeting rooms and editorial offices, for the purposes of organizing information about chemical substances, in order to support research and development within the turn of the century synthetic chemicals industry (Hepler-Smith 2015a; Hepler-Smith 2015b; Hepler-Smith 2016). And both making and maintaining this system of molecular identity took (and takes) a whole lot of *work*.
My intention in juxtaposing these images is to direct historical and ethnographic attention to the situated practices—including especially bureaucratic practices—through which the simplicities, complexities, and uncertainties of environmental toxicity are constituted. They are typically taken to be baked into the material nature of chemical substances and bodies. To a certain degree, they are; but they are also the product of a way of ordering the world of substances that I am calling “molecular bureaucracy” (Hepler-Smith 2019). The institutions, practices and technologies that render the world molecular (cf. Myers 2015) entail simplifications, frustrations, fortuitous affordances, and regrettable consequences. Training our vision on easily-overlooked legal definitions, information technologies, administrative procedures, clerical labor, and nomenclature conventions can help shed light on why toxic subjects get visualized in the ways they do.
References:
Hepler-Smith, Evan. 2015. "'Just as the Structural Formula Does': Names, Diagrams, and the Structure of Organic Chemistry at the 1892 Geneva Nomenclature Congress." Ambix 62 (1): 1-28. doi: 10.1179/1745823414Y.0000000006.
Hepler-Smith, Evan. 2015b. “Systematic Flexibility and the History of the IUPAC Nomenclature of Organic Chemistry.” Chemistry International 37 (2): 10-14. doi: 10.1515/ci-2015-0232.
Hepler-Smith, Evan. 2016. “Changing Names and Naming Change: Transformations in the ‘International Machinery’ of Chemical Information.” Proceedings of the International Workshop on the History of Chemistry, March 2-4 2015, Tokyo. 68-76. http://kagakushi.org/iwhc2015/proceedings/.
Hepler-Smith, Evan. 2019. "Molecular Bureaucracy: Toxicological Information and Environmental Protection." Environmental History 24 (3). Forthcoming.
Myers, Natasha. 2015. Rendering Life Molecular: Models, Modelers, and Excitable Matter. Durham: Duke University Press.
Scott, Janet D. 1938. “The Chemist at Work. XIV. My Work with Chemical Abstracts.” Journal of Chemical Education 15 (6): 271–75. doi: 10.1021/ed015p271.

Evan Hepler-Smith. 26 November 2018, "Visualizing the chemistry of regret", Center for Ethnography, Platform for Experimental Collaborative Ethnography, last modified 9 May 2019, accessed 1 February 2025. http://centerforethnography.org/content/visualizing-chemistry-regret


Substantive Caption:
The main image depicts the editorial offices of Chemical Abstracts, circa 1938. For most of the 20th century and through the present-day, the abstract journals, collective indexes, and databases of Chemical Abstracts (now Chemical Abstracts Service, or CAS) have been the authoritative source for bibliographic and regulatory information about chemical substances and the chemical sciences, including allied fields such as toxicology. In this image, “index workers” are engaged in compiling an annual or decennial index of the publication. In the early 20th century, the new specialization of "literature chemistry," demanding considerable chemical expertise, presented professional opportunities seized by chemists belonging to groups marginalized within lab-based chemical occupations--most prominently, women.
The vast majority of these cards refer to individual chemical substances rather than subject headings or author names. Much index labor involved writing, rewriting, cross-referencing, and error-checking the systematic chemical names that were the key to the whole enterprise, since they allowed for the arrangement of hundreds of thousands (today hundreds of millions) of substances in chemically-meaningful alphabetical order. This work was particularly challenging because the research chemists who wrote the journal articles abstracted and indexed in Chemical Abstracts often did not themselves use systematic names. This was both because the names were cumbersome and because the uniform chemical ontology that they expressed was sometimes a poor fit for how chemists defined the substances that their research addressed. Sustaining the molecular ontology of chemistry and chemists' access to information was crucial, challenging work.
“Among personal characteristics for this type of work accuracy perhaps should rank highest, and with that conscientiousness, patience, a meticulous attentiveness to detail (without a loss of perspective of relative values, since a great deal of work has to be turned out, at times under considerable pressure), power of concentration, good judgment, and interest in words as words, a love of puzzles and of guessing and digging out elusive ideas, meanings, words, and formulas. An interest in things rather than people is likely to lead to greater satisfaction in this type of work, since the opportunities for personal professional contacts outside the office are relatively few. The analytical rather than the creative type is probably best suited to this kind of work. Good eyes (at least strong ones) are absolutely essential, while the ability to sit hour after hour without much relief is a requirement not to be laughed off. Work of this nature is not for the overly energetic or restless person” (Scott 1938, 275; italics in original).
The inset image depicts a form of the sort that was used when CAS re-created this whole operation on digital computers during the 1960s.
Molecular identity is not given by nature. Rather, it was made in meeting rooms and editorial offices, for the purposes of organizing information about chemical substances, in order to support research and development within the turn of the century synthetic chemicals industry (Hepler-Smith 2015a; Hepler-Smith 2015b; Hepler-Smith 2016). And both making and maintaining this system of molecular identity took (and takes) a whole lot of *work*.
My intention in juxtaposing these images is to direct historical and ethnographic attention to the situated practices—including especially bureaucratic practices—through which the simplicities, complexities, and uncertainties of environmental toxicity are constituted. They are typically taken to be baked into the material nature of chemical substances and bodies. To a certain degree, they are; but they are also the product of a way of ordering the world of substances that I am calling “molecular bureaucracy” (Hepler-Smith 2019). The institutions, practices and technologies that render the world molecular (cf. Myers 2015) entail simplifications, frustrations, fortuitous affordances, and regrettable consequences. Training our vision on easily-overlooked legal definitions, information technologies, administrative procedures, clerical labor, and nomenclature conventions can help shed light on why toxic subjects get visualized in the ways they do.
Design Statement:
This is a pairing of two images, one copied from a chemical journal article published in 1938, one a photograph of manuscript material from an archive.
The main image and the article from which it is drawn, written by an editor at the publication Chemical Abstracts, describe the enormous labor of keeping this foundational, taken-for-granted information resource up to date. It is an exercise in infrastructural inversion (Star and Bowker 1999) avant la lettre. The inset image, the author’s photograph of a manuscript page produced circa 1960, depicts a notation designed by an information entrepreneur to enable the application of mechanical and digital tools to the sort of work illustrated in the main images.
The main image anchors databases and the mechanical objectivity that they embody (Porter 1995, Daston and Galison 2007) in the material and social world of information labor. The inset image exemplifies an effort to shift the social location of judgment and expertise from people intimately engaged with a specific database to machine- and algorithm-makers. Ethnographers and historians can and should ask what knowledge and what social relations, embodied in both people and artifacts, lie behind their databases and visualization methods. Sometimes, the answer may be relatively epistemologically and ethically insignificant vis-à-vis the story at issue; other times, the answer may be the story (e.g. Radin 2017).
References:
Bowker, Geoffrey C., and Susan Leigh Star. 1999. Sorting Things Out: Classification and Its Consequences. Cambridge, MA: MIT Press.
Daston, Lorraine and Peter Galison. 2007. Objectivity. New York: Zone Books.
Hepler-Smith, Evan. 2015. "'Just as the Structural Formula Does': Names, Diagrams, and the Structure of Organic Chemistry at the 1892 Geneva Nomenclature Congress." Ambix 62 (1): 1-28. doi: 10.1179/1745823414Y.0000000006.
Hepler-Smith, Evan. 2015b. “Systematic Flexibility and the History of the IUPAC Nomenclature of Organic Chemistry.” Chemistry International 37 (2): 10-14. doi: 10.1515/ci-2015-0232.
Hepler-Smith, Evan. 2016. “Changing Names and Naming Change: Transformations in the ‘International Machinery’ of Chemical Information.” Proceedings of the International Workshop on the History of Chemistry, March 2-4 2015, Tokyo. 68-76. http://kagakushi.org/iwhc2015/proceedings/.
Hepler-Smith, Evan. 2019. "Molecular Bureaucracy: Toxicological Information and Environmental Protection." Environmental History 24 (3). Forthcoming.
Myers, Natasha. 2015. Rendering Life Molecular: Models, Modelers, and Excitable Matter. Durham: Duke University Press.
Porter, Theodore M. 1995. Trust in Numbers: The Pursuit of Objectivity in Science and Public Life. Princeton: Princeton University Press.
Radin, Joanna. 2017. “Digital Natives”: How Medical and Indigenous Histories Matter for Big Data. Osiris 32 (1): 43–64.
Scott, Janet D. 1938. “The Chemist at Work. XIV. My Work with Chemical Abstracts.” Journal of Chemical Education 15 (6): 271–75. doi: 10.1021/ed015p271.
(Revision of May 9, 2019)